Role of Oxygen in Combustion, How Oxygen Makes Fires Grow and Why It Becomes Dangerous
In real fire incidents, oxygen is rarely discussed until it is too late. Fire investigations repeatedly show that fires grow out of control not only because fuel and heat are present, but because oxygen is allowed to flow freely or becomes unintentionally enriched.
According to HSE fire and explosion guidance, oxygen is a critical element in combustion, as it supports and sustains the chemical reaction of fire.
This guide explains the role of oxygen in combustion from a practical fire behaviour and prevention perspective, focusing on how oxygen affects fire growth, where oxygen related failures occur, and how controlling oxygen reduces fire severity.
Why Oxygen Is the Most Misunderstood Fire Element
Fuel and heat are easy to see. Oxygen is invisible, silent, and usually taken for granted.
In most environments:
- Oxygen is always available
- Ventilation systems continuously supply fresh air
- Doors, windows, and ducts feed fire growth
When oxygen supply is uncontrolled, fires grow rapidly and become difficult to contain.
How Oxygen Actually Supports Fire
Fire occurs when heated fuel vapours react with oxygen and release heat.
In practical terms:
- More oxygen means faster burning
- Faster burning produces more heat
- More heat releases more fuel vapours
- Fire growth accelerates rapidly
This feedback loop explains why fires become uncontrollable once ventilation increases.
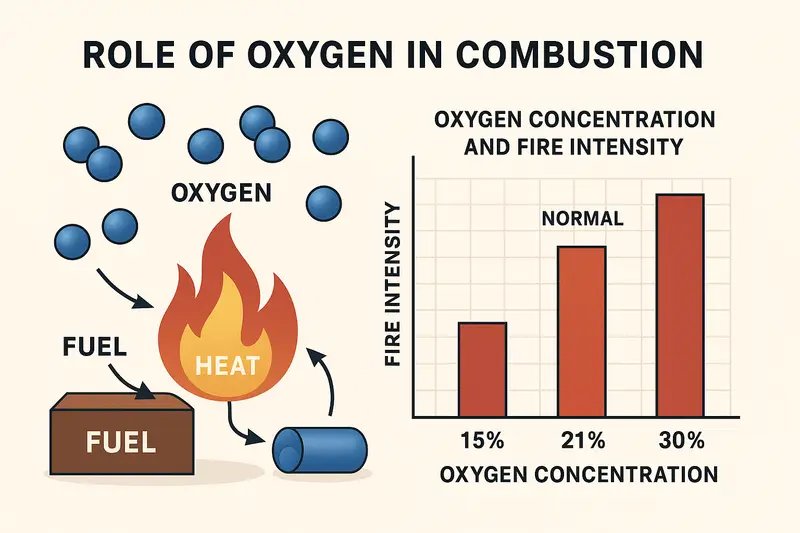
Oxygen Levels and Real Fire Behaviour
Low Oxygen Conditions
When oxygen drops due to fire consumption:
- Flames weaken
- Smoke increases
- Combustion becomes incomplete
- Large amounts of toxic gases are produced
This often happens in sealed rooms, basements, and confined spaces.
Danger
Unburned fuel vapours accumulate, creating explosion risk if oxygen suddenly enters.
Normal Oxygen Conditions
At normal air concentration:
- Fires behave predictably
- Flame spread follows fuel and ventilation patterns
- Heat release remains manageable in early stages
Most fire safety systems are designed for this condition.
Oxygen Enriched Conditions, The Most Dangerous Scenario
When oxygen concentration increases:
- Materials ignite more easily
- Flames spread extremely fast
- Fire intensity rises sharply
- Even materials that normally resist burning can ignite
Oxygen enriched conditions occur more often than people realize.
Common locations
- Hospitals using medical oxygen
- Welding and cutting areas
- SCBA filling rooms
- Industrial oxygen pipelines
Many severe fires are caused not by sparks, but by oxygen enrichment.
How Oxygen Changes Ignition Risk
In oxygen rich environments:
- Ignition temperature drops
- Small sparks cause flash fires
- Static electricity becomes dangerous
In oxygen poor environments:
- Ignition becomes difficult
- Fire may smoulder
- Explosion risk increases if oxygen re-enters suddenly
Both extremes are dangerous in different ways.
Oxygen and Fire Growth Inside Buildings
Confined Space Fires
In sealed rooms:
- Fire quickly consumes oxygen
- Flames may die down
- Temperature remains high
- Unburned vapours accumulate
Opening a door suddenly allows oxygen to rush in, causing backdraft or flash fire.
This is one of the deadliest fire scenarios.
Ventilated Fires
When doors, windows, or ventilation systems feed oxygen:
- Fire grows rapidly
- Heat release increases
- Flashover becomes likely
- Fire spreads beyond the room of origin
Many fires escalate because ventilation was uncontrolled.
Oxygen Related Hazards People Commonly Create
Fire investigations repeatedly identify these failures:
- Leaving doors open during fires
- Using fans that feed oxygen to flames
- Oxygen leaks in hospitals and welding areas
- Oil or grease contamination on oxygen equipment
- Poor ventilation design around oxygen systems
These hazards often exist unnoticed until ignition occurs.
Oxygen and Firefighting Decisions
Firefighters and trained responders actively control oxygen.
Common techniques include:
- Closing doors to limit air flow
- Using ventilation strategically
- Smothering fires with foam or CO₂
- Flooding spaces with inert gases
- Cooling hot gases to reduce oxygen demand
Firefighting is as much about oxygen control as it is about extinguishing flames.
Oxygen Reduction Fire Protection Systems
Some facilities intentionally lower oxygen levels to prevent fire.
Used in:
- Data centers
- Archives and libraries
- Control rooms
- Sensitive manufacturing areas
These systems keep oxygen just low enough to prevent ignition while remaining safe for people.
Oxygen and Clothing, A Hidden Fire Risk
Oxygen enriched air can saturate fabrics.
Consequences:
- Clothing ignites instantly
- Fire spreads over the body rapidly
- Burns are severe even from small sparks
This risk is common in medical facilities and welding operations.
Practical Oxygen Control Measures That Prevent Fires
Engineering Controls
- Leak detection on oxygen systems
- Proper ventilation design
- Oxygen compatible materials only
- Alarm systems for oxygen enrichment
Administrative Controls
- Clear procedures for oxygen handling
- Permit systems for oxygen related work
- Training on oxygen fire risks
- Signage in oxygen enriched areas
Safe Work Practices
- Keep oil and grease away from oxygen equipment
- Never modify oxygen fittings
- Avoid synthetic clothing in oxygen areas
- Shut oxygen supply when not in use
Inspection Focus Areas for Oxygen Hazards
During inspections, focus on:
- Oxygen cylinder storage
- Pipeline leak signs
- Ventilation effectiveness
- Door and compartment control
- Housekeeping around oxygen equipment
Ignoring oxygen hazards leads to rapid fire escalation.
Who Should Use This Guide
This guide is intended for:
- Safety officers
- Facility managers
- Fire wardens
- Maintenance supervisors
- Employers responsible for fire prevention
Conclusion
Oxygen does not cause fires by itself, but it determines how fast fires grow, how intense they become, and whether they turn catastrophic. Fires escalate when oxygen is uncontrolled, enriched, or suddenly introduced into hot, fuel rich environments.
Effective fire prevention is not only about removing heat and fuel. It also requires controlling oxygen flow, enrichment, and ventilation.
Understanding oxygen behaviour turns fire safety from theory into real prevention.
Fire Triangle Explained: Definition, Elements, Examples and Importance
Heat Sources in Industrial Fires: Causes, Risks, Control Measures and Prevention
